
Custom Enclosures for Medical & Life-Science Equipment
From compact devices to large systems, built to meet the strictest healthcare standards. Discover the different methods to manufacture medical enclosures and learn how to choose the one that’s right for your project.
Years of experience
Projects per year
Countries, countless satisfied customers
Production methods
Plastic and metal materials
Why choose Protolis
Medical standards and compliance
Protolis follows the highest industry standards, complying with ISO 9001 & ISO 13485, FDA, and UL 94 requirements for medical-grade enclosures.

High-Quality Aesthetics
We offer a wide range of finishes that give your medical devices a professional appearance - hospital-grade disinfectant-resistant coatings, logo printing, copper EMI shielding.

Optimized costs and lead time
We offer cost-effective solutions without compromising on quality. For urgent projects, our rapid prototyping service ensures parts are delivered within weeks.


About Protolis
At Protolis, we combine engineering precision with aesthetic excellence to deliver high-quality plastic and metal housings for medical and life science equipment. Whether through CNC machining, sheet metal fabrication, injection molding, or vacuum casting, we deliver tailored solutions from small prototypes to full systems.
Medical housing and enclosure applications
Our custom enclosures are used in critical medical applications, including patient monitoring systems (ECG, pulse oximeters, ventilators), imaging devices (X-ray, MRI, ultrasound), wearable medical devices, and laboratory equipment.




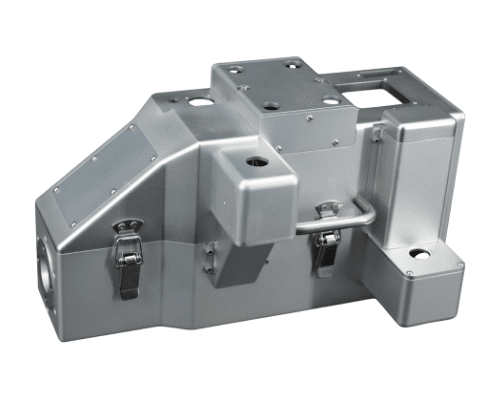
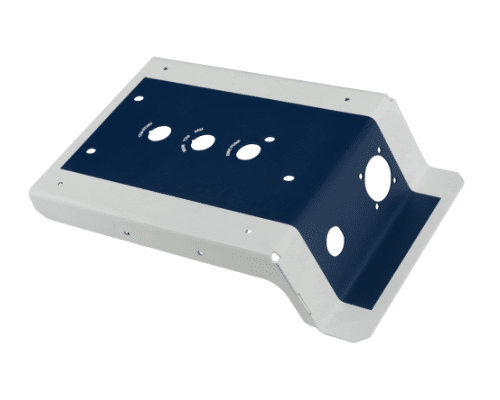

With over 15 years in medical enclosure manufacturing, we help you choose the most efficient, compliant, and cost-effective production path from prototyping to small-batch runs.
Frequently Asked Questions
Medical device enclosures are made from high-performance materials chosen for durability, sterilization resistance, and compliance with medical regulations.
Polycarbonate (PC) is widely used for its impact resistance and transparency, ideal for imaging and diagnostic equipment.
ABS is a lightweight, cost-effective option for electronic device housings.
PEEK is preferred for its biocompatibility and high-temperature resistance, making it suitable for demanding applications.
Medical-grade polypropylene (PP) is chosen for its chemical resistance and ease of sterilization.
For stronger enclosures, stainless steel (316L) offers corrosion resistance, while aluminum provides lightweight strength with EMI shielding for electronic devices.
Titanium is ideal for implantable or surgical enclosures due to its biocompatibility and durability.
All materials meet ISO 13485, IEC 60601, and FDA standards for medical applications. Let us help you choose the best material for your medical enclosure needs.
Yes, we offer low-volume production using CNC machining, vacuum casting (or reaction injection molding), and injection molding for medical device prototyping and limited series.
Lead times depend on design complexity, material choice, and production method, but we offer rapid prototyping and short-run production to meet tight deadlines.
Clients Feedback



Let’s Build Your Ideal Enclosure!
Download our manufacturing guide and discover the proven methods we use to create high-quality custom enclosures