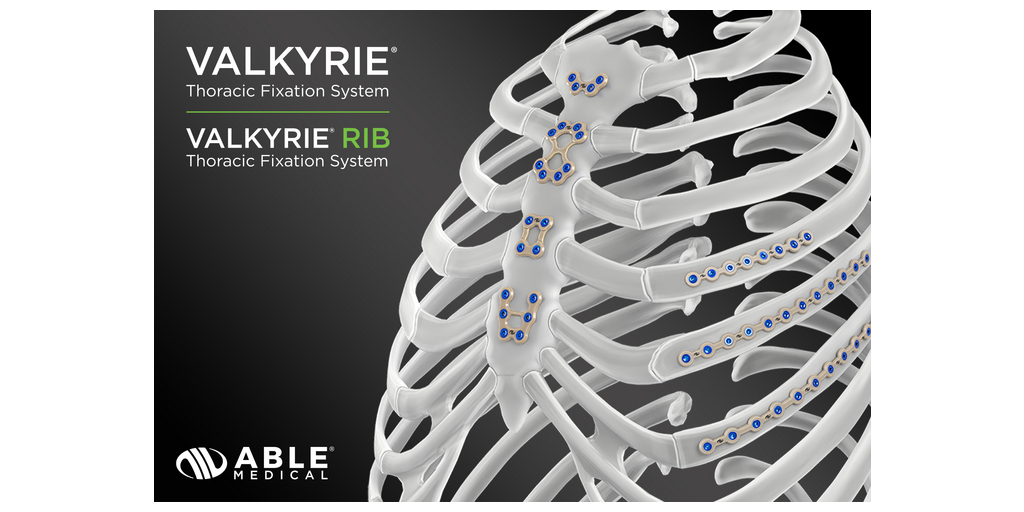
- Able Medical exceeds 10,000 U.S. procedures since the release of the Valkyrie® Thoracic Fixation System.*
- Over 150 hospitals use the new Valkyrie Thoracic Fixation System, consistently representing competitive conversions and repeat users.
- Valkyrie Thoracic Fixation System is the market’s first single-use radiolucent plating system.
MARQUETTE, Mich.–(BUSINESS WIRE)–#PEEKplating–Able Medical, a leading global medical device company focused on sternal fixation, announced the completion of the 10,000th procedure in the U.S. with the Valkyrie Thoracic Fixation System. The system’s unique PEEK material allows the implant to be contoured in-situ, which saves time and improves efficiency in the operating room by reducing procedural steps.
The Valkyrie Thoracic Fixation System includes three key components: a sterile-packaged PEEK plate, sterile-packaged screws, and a sterile-packaged driver. Its adaptive material provides intraoperative flexibility, allowing surgeons to select the optimal implant for each patient’s anatomy. With streamlined instrumentation focused on speed, efficiency, and simplicity, the system supports a smooth surgical workflow. Most new surgeon adopters have converted from competitive systems and continue to be repeat users.
“The rapid uptake, the acceleration of new adopting surgeons, and the repeat usage all demonstrate the value that the Valkyrie System brings to surgeons,” said Jeffrey Gilhool, director of marketing for Able Medical. “Offering implant material that can better match the patient’s anatomy is unique to the system and has clearly been viewed as a benefit. The fact that the 10,000-procedure milestone was achieved so quickly indicates that not only is Valkyrie delivering value to surgeons and patients but that the adoption of rigid fixation is accelerating.”
Able Medical’s portfolio is unique in the utilization of PEEK material in sternal closure, offering both sternal and thoracic implants with differentiated designs. The Valkyrie system delivers both clinical and economic value to its consumers. Its ability to individualize itself to each specific patient and its single use design reduces waste to the healthcare system while maximizing patient benefits.
* Data on file.
About Able Medical Devices
Able Medical Devices, headquartered in Marquette, Michigan, is a global partner to the orthopedic and broader medical device industry. As a subsidiary of J.M. Longyear, a privately held Michigan-based investment and asset management company, Able Medical benefits from a strong legacy of operational excellence and long-term investment support.
The company is recognized as a leader in cardiothoracic products, including thoracic fixation and sternal closure solutions. Able Medical’s expertise extends across spine, trauma and cardiothoracic applications, where it works closely with surgeons to bring precision-engineered, next-generation solutions to market.
For more information, visit www.AbleMedicalDevices.com.
Contacts
Media Contact
Jeffrey Gilhool
Senior Director of Marketing
Able Medical Devices
Email: [email protected]
Phone: (906) 373-9539
Website: www.AbleMedicalDevices.com