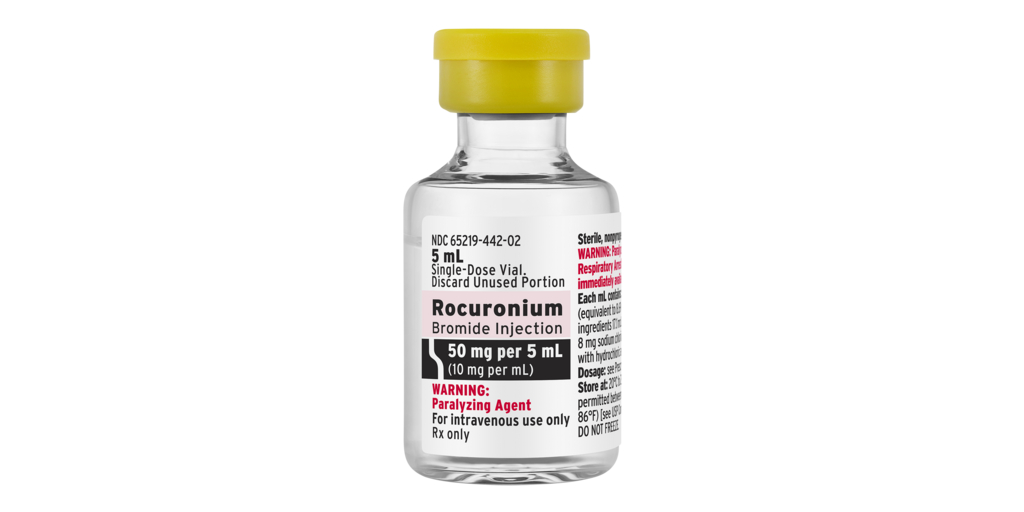
New product requires no refrigeration
LAKE ZURICH, Ill.–(BUSINESS WIRE)–#CommittedToLife–Fresenius Kabi, an Operating Company of Fresenius, and a leading provider of essential medicines and medical technologies, announced today it has launched Rocuronium Bromide Injection Room Temperature Stable (RTS), the first formulation of Rocuronium Bromide Injection that does not require refrigeration.
Rocuronium Bromide Injection RTS was formulated to be stored at room temperature, eliminating the need for refrigeration, which is expected to streamline logistics.
Rocuronium Bromide Injection is a nondepolarizing neuromuscular blocking agent used as an adjunct to general anesthesia to facilitate tracheal intubation and to provide skeletal muscle relaxation during surgery or mechanical ventilation.
Because it can be stored at room temperature, Rocuronium Bromide Injection RTS is expected to reduce the cost and complexity of transportation and storage because a cold supply chain is not needed. Hospital pharmacies can store Rocuronium Bromide Injection RTS at room temperature, which can free up refrigerated space for other medications.
Rocuronium Bromide Injection RTS is formulated, filled, and packaged in the United States at Fresenius Kabi’s facility in Grand Island, New York. Since 2017, Fresenius Kabi has invested nearly $1 billion to expand and modernize advanced U.S. pharmaceutical production and distribution facilities. More than 70% of the units shipped by Fresenius Kabi in the U.S. are drugs listed on the FDA’s Essential Medicines List.1 Learn more at moreinamerica.com.
“The introduction of Rocuronium Bromide Injection RTS highlights our leadership in providing innovative solutions that help customers improve efficiency and deliver quality care,” said Joel Rosenstack, president, U.S. Pharmaceuticals. “Our strong commitment to U.S. manufacturing helps ensure a reliable supply for patients and clinicians.”
Fresenius Kabi’s Rocuronium Bromide Injection RTS is available in two presentations: 50 mg per 5 mL and 100 mg per 10 mL.
IMPORTANT SAFETY INFORMATION
CONTRAINDICATIONS
Rocuronium Bromide Injection is contraindicated in patients known to have hypersensitivity (e.g., anaphylaxis) to rocuronium bromide or other neuromuscular blocking agents.
WARNINGS AND PRECAUTIONS
- Appropriate Administration and Monitoring: Use only if facilities for intubation, mechanical ventilation, oxygen therapy, and an antagonist are immediately available.
- Anaphylaxis: Severe anaphylaxis has been reported. Consider cross-reactivity among neuromuscular blocking agents.
- Risk of Death due to Medication Errors: Accidental administration can cause death.
- Need for Adequate Anesthesia: Must be accompanied by adequate anesthesia or sedation.
- Residual Paralysis: Consider using a reversal agent in cases where residual paralysis is more likely to occur.
ADVERSE REACTIONS
- Most common adverse reactions (2%) are transient hypotension and hypertension.
To report SUSPECTED ADVERSE REACTIONS, contact Fresenius Kabi USA, LLC at 1-800-551-7176, option 5, or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- Succinylcholine: Use before succinylcholine has not been studied.
- Nondepolarizing muscle relaxants: Interactions have been observed.
- Enhanced Rocuronium Bromide Injection activity possible: Inhalation anesthetics, certain antibiotics, quinidine, magnesium, lithium, local anesthetics, procainamide.
- Reduced Rocuronium Bromide Injection activity possible: Anticonvulsants.
USE IN SPECIFIC POPULATIONS
- Pregnancy: Not recommended for rapid sequence induction in patients undergoing Cesarean section.
- Pediatric Use: Onset time and duration will vary with dose, age, and anesthetic technique. Not recommended for rapid sequence intubation in pediatric patients.
INDICATIONS AND USAGE
Rocuronium Bromide Injection is a nondepolarizing neuromuscular blocking agent indicated as an adjunct to general anesthesia to facilitate both rapid sequence and routine tracheal intubation, and to provide skeletal muscle relaxation during surgery or mechanical ventilation.
This Important Safety Information does not include all the information needed to use Rocuronium Bromide Injection safely and effectively. Please see Full Prescribing Information for Rocuronium Bromide Injection at www.fresenius-kabi.com/us.
REFERENCE
1. 2023 Data on file; https://www.fda.gov/media/143406/download
About Fresenius Kabi
As a global healthcare company, Fresenius Kabi is Committed to Life. The company’s products, technologies, and services are used for the therapy and care of patients with critical and chronic conditions. With more than 41,000 employees and present in more than 100 countries, Fresenius Kabi’s expansive product portfolio focuses on providing access to essential medicines and technologies.
In Biopharma, Fresenius Kabi offers cutting-edge biosimilars for autoimmune diseases and oncology. With leading market positions in Clinical Nutrition, a broad portfolio of enteral and parenteral products makes a distinct difference in patients’ nutritional status. In MedTech, the company provides vital infusion pumps, cell and gene therapy devices, disposables, and more. Fresenius Kabi is a global leader in supplying blood collection bags and devices, supporting blood banks and health care facilities worldwide. The company’s I.V. Generics and Fluids for infusion therapy help save millions of lives every year, in emergency medicine, surgery, oncology, and intensive care.
Fresenius Kabi takes a holistic approach to health care and uniquely combines experience, expertise, innovation, and dedication – making a difference in the lives of 450 million patients annually. With the #FutureFresenius strategy, the company is developing, producing, and selling new products and technologies and aspires to expand its position as a leading global provider of therapies, improve patient care, generate sustainable value for stakeholders – shaping the future of healthcare.
Fresenius Kabi is part of the Fresenius Group, founded in 1912, along with Helios and Quirónsalud. As ONE team, the companies in the Fresenius Group are committed to providing lifesaving and life-changing healthcare solutions on a global scale.
For more information, please visit www.fresenius-kabi.com/us. To learn about U.S. career opportunities at Fresenius Kabi, visit us at www.fresenius-kabi.com/us/careers and follow us on LinkedIn and Facebook.
This release contains forward-looking statements that are subject to various risks and uncertainties. Future results could differ materially from those described in these forward-looking statements due to certain factors, e.g., changes in business, economic and competitive conditions, regulatory reforms, results of clinical trials, foreign exchange rate fluctuations, uncertainties in litigation or investigative proceedings, and the availability of financing. Fresenius Kabi does not undertake any responsibility to update the forward-looking statements in this release.
Contacts
Media contact
Joanie Clougherty (614) 717-5741
[email protected]