$11.6M Department of Defense-supported pivotal study evaluates rapid, personalized, drug-free neuromodulation therapy for postpartum depression
BURLINGAME, Calif.–(BUSINESS WIRE)–#PPD—Magnus Medical, Inc., a pioneering therapeutic neuromodulation company advancing precision medicine in brain health, today announced enrollment of the first patient at University of Massachusetts Chan Medical School (UMass Chan), a leading academic health system, in its U.S. Department of Defense (DoD)-funded, multi-center pivotal clinical trial designed to evaluate the safety and effectiveness of SAINT® neuromodulation therapy for the treatment of postpartum depression (PPD).
The milestone marks the operational launch of the $11.6 million randomized controlled trial (RCT), which will test whether SAINT, an FDA-cleared, non-invasive treatment for major depressive disorder (MDD), can provide rapid, safe and durable symptom relief for women experiencing depression following childbirth. SAINT was commercially launched in April 2024 following clinical studies demonstrating rapid, robust symptom relief and remission in people with treatment-resistant MDD.
Unlike prior studies of SAINT therapy, this trial does not require prior antidepressant or psychotherapy treatment failure. Eligible participants include women up to 12 months postpartum with moderate to severe depression, including those receiving stable antidepressant medication or psychotherapy.
“The first patient enrolled at UMass Chan represents an important step forward in translating precision neuromodulation to a population with urgent, unmet needs,” said Brandon Bentzley, M.D. Ph.D., co-founder and chief scientific officer of Magnus Medical, who serves as the study’s principal investigator. “We are deeply honored by this support from the Department of Defense, as postpartum depression is devastating, yet today’s most commonly used treatments are often too slow or too burdensome for new mothers. SAINT therapy has already shown the ability to rapidly lift depressive symptoms without systemic side effects, offering hope for a safer, faster solution.
“Importantly, this trial allows us to rigorously test SAINT as a non-drug intervention for postpartum depression and to move closer to a future where mental health care is both more precise and more effective,” continued Dr. Bentzley. “It also supports the DoD’s mission to advance innovative therapies for vulnerable populations, including new mothers in military families and beyond.”
Postpartum depression affects approximately 500,000 women in the U.S. annually, and rates of PPD have increased substantially since 2000.1 PPD can severely impact maternal well-being and the early bonding period between mother and child. Current treatments, including antidepressants and psychotherapy, are often slow to act and may not be effective for all women. Antidepressants may also pass to the infant during breastfeeding, causing many new mothers to be reluctant to take them.
The trial is actively enrolling up to 192 women aged 18-45 years, all diagnosed with a major depressive episode (MDE) with peripartum onset, the technical diagnosis for postpartum depression. The study is being conducted at four leading research institutions: the Medical University of South Carolina (MUSC), Dell Medical School at The University of Texas at Austin (Dell Med), the Icahn School of Medicine at Mount Sinai and UMass Chan. Additional information regarding trial enrollment at the four leading research institutions may be found at learn.magnusmedical.com/ppd-trial/.
This work is supported by the Assistant Secretary of Defense for Health Affairs, endorsed by the Department of Defense, in the amount of $11,619,193.00 through the Peer Reviewed Medical Research Program under Award Number HT94252510222. Opinions, interpretations, conclusions, and recommendations are those of the author(s) and are not necessarily endorsed by the Assistant Secretary of Defense for Health Affairs or the Department of Defense.
About SAINT
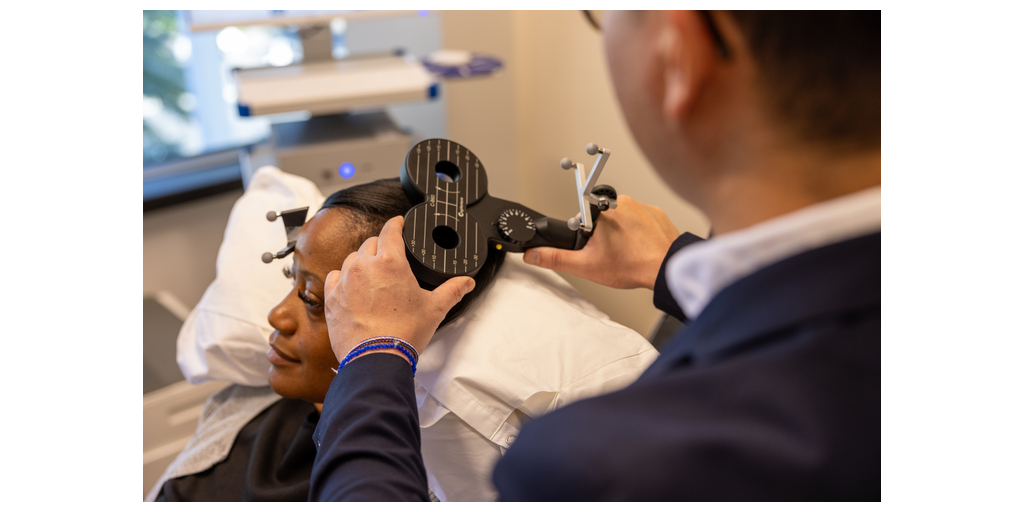
The SAINT neuromodulation platform is the first and only FDA-cleared treatment for major depressive disorder (MDD) that uses functional MRI data to guide personalized, non-invasive neuromodulation over an accelerated five-day schedule, with stimulation precisely targeted to brain regions identified through each person’s unique connectivity patterns. SAINT has been designated a Breakthrough Device by the FDA and is the first mental health therapy to receive innovation support from the Centers for Medicare & Medicaid Services through the New Technology Add-on Payment (NTAP) and New Technology Ambulatory Payment Classification (APC) programs. Foundational studies have shown that 79% of patients with major depression achieved remission in an average of just 2.6 days. Clinical trials are ongoing to evaluate SAINT therapy for other neuropsychiatric conditions, including obsessive-compulsive disorder, bipolar depression, and now postpartum depression. These indications have not been cleared by the FDA.
About Magnus Medical
Magnus Medical, Inc., is a privately held therapeutic neuromodulation company co-founded in 2020 to advance precision medicine in brain health. Its platform technology combines neuroimaging, proprietary targeting algorithms and individualized neurostimulation to address complex brain conditions. Magnus’ first product, the SAINT neuromodulation system, is FDA-cleared for adults with major depressive disorder. Building on this foundation, the company is expanding the SAINT platform to explore new indications and unlock more personalized approaches to brain health. For more information, visit https://www.magnusmedical.com.
|
____________________ |
| 1 |
Contacts
Media Contact:
Amy Cook
Media resources
[email protected]