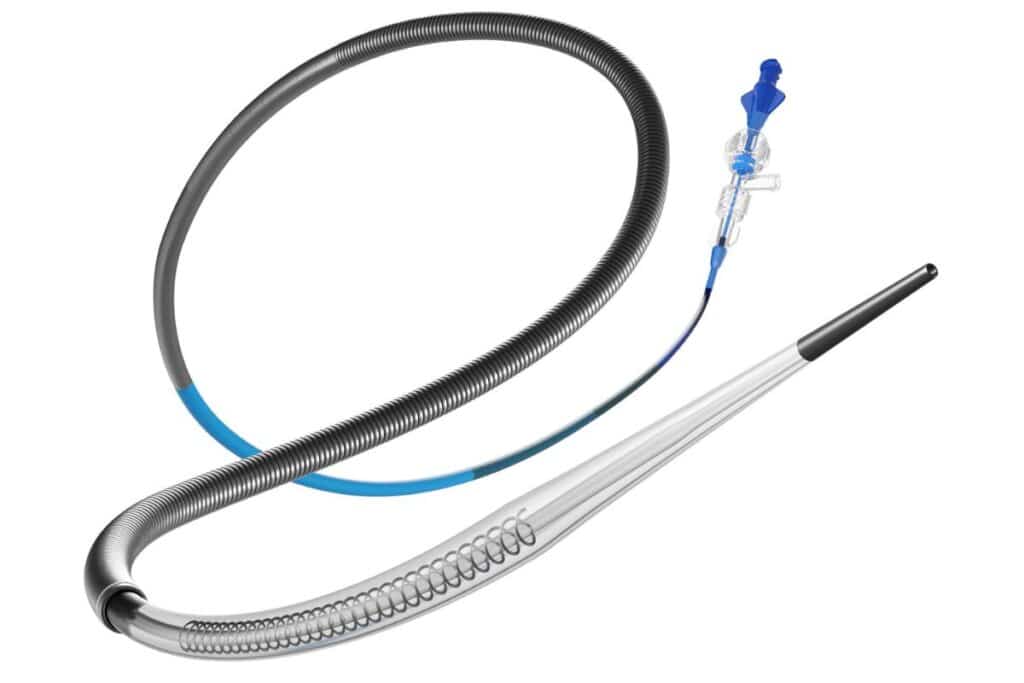
The U.S. Food and Drug Administration (FDA) has granted clearance to Perfuze’s Zipline, a single-use access catheter designed for acute ischemic stroke (AIS) procedures. This device facilitates the delivery of large-bore catheters using standard endovascular techniques. During AIS interventions, Zipline is navigated over a guidewire to the targeted area within the neurovasculature, allowing an outer catheter to be advanced over it to the desired location. Perfuze asserts that Zipline’s enhanced support and navigational ease optimize clot removal efficiency, potentially leading to improved procedural success rates and patient outcomes.
Dr. Jay Dolia, an assistant neurology professor at Emory University School of Medicine, shared his experience with Zipline, noting that it “enabled rapid clot access and aspiration, even in complex anatomy.”
In conjunction with the FDA clearance, Perfuze has secured a €22 million ($23.9 million) follow-on funding round, elevating its total funding to approximately €50 million. Led by existing investors such as EQT Life Sciences, Seroba, and SV Health, this financing aims to support the limited market release of the Zipline and Millipede catheters to select U.S. stroke centers. Additionally, the funds will advance research and development initiatives to further expand Perfuze’s stroke treatment portfolio.
Acute ischemic stroke, accounting for 85% of all strokes, occurs when blood flow to the brain is obstructed, typically by blood clots or arterial plaque buildup (atherosclerosis). Despite its prevalence, fewer than 30% of patients receive treatment within the optimal timeframe, underscoring the need for enhanced education on recognizing AIS symptoms.